Global assembling of Academicians, Researchers, Scholars & Industry to disseminate and exchange information at 100+ Allied Academies Conferences
ession on: PreclinicalStudies
Concluding whether the drug molecule is ready for Clinicaltrials starts from the pre clinical studies Drug testing in Invitro and In vivo animal studies are done to ensure the safety and efficacy of the drug. MSRD will be evaluated in the preclinical studies, thespecies of the selected animals are identified, and testing is carried out with the proper approval from the government bodies. Most of animal specimens usedare Mice, rats, Guinea pigs. Pharmacokineticand pharmacodynamic studies are checked in animal studies
Related: Clinical Trials Conferences| Clinical TrialConferences | Pharmacovigilance Conferences | Clinical Trials Congress |ClinicalTrials Meetings | MedicalConferences | PharmacologyConferences | ClinicalResearch Conferences | Clinical research Society | Clinical Research Associations
Recommended: 9th World Congress on Clinical Pharmacyand Pharmacy Practice Amsterdam, Netherlands March 25-26, 2019, 4thInternational Conference onMedical Microbiology 2019 Vienna, Austria May 20-21, 2019, CPD Accredited8th European ClinicalMicrobiology and ImmunologyCongress Edinburgh, Scotland June 12-13, 2019, International Conference on Clinical Trials& Pharmacovigilance,Prague Czech Republic, September 23 -24 2019. World Congress on Biomarkers andClinical Research, Tokyo, Japan, August 5-6, 2019.
Related Associations: BelgianSociety of Pharmaceutical Sciences (BGFW), EuropeanFederation for Pharmaceutical Sciences (EUFEPS),ItalianSociety for Pharmaceutical Sciences (SISF), Associationof the British Pharmaceutical Industry (ABPI), InternationalPharmaceutical Federation (FIP)
Session on: ClinicalTrials
The target of Clinical trials is to determine the safety of drug and dosage in the participant. Exploring ways fora comfortable life for people with chronic illness. Clinical Trials are conducted by a team of researcher which includes a doctor, social workers, and other healthcare professionals. Clinical Trials done in the United States must be approved and monitored by the Institutional Review Board.
Related: Clinical Trials Conferences| Clinical TrialConferences | Pharmacovigilance Conferences | Clinical Trials Congress |ClinicalTrials Meetings | MedicalConferences | PharmacologyConferences | ClinicalResearch Conferences | Clinical research Society | Clinical Research Associations
Recommended: 9th World Congress on Clinical Pharmacy and Pharmacy Practice Amsterdam, Netherlands March 25-26, 2019, 4thInternational Conference onMedical Microbiology 2019 Vienna, Austria May 20-21, 2019, CPD Accredited8th European Clinical Microbiology and Immunology Congress Edinburgh, Scotland June 12-13, 2019, International Conference on Clinical Trials& Pharmacovigilance,Prague Czech Republic, September 23 -24 2019. World Congress on Biomarkers andClinical Research, Tokyo, Japan, August 5-6, 2019.
Related Associations: CanadianSociety for Pharmaceutical Sciences (CSPS), PharmaceuticalManufacturers Association of Canada (PMAC),AmericanAssociation of Pharmacy Technicians (AAPT), AmericanSociety of Health-System Pharmacists (ASHYP)| SpanishSociety of Pharmaceutics and PharmaceuticalTechnology(SEFC).
Session on: ClinicalToxicology
The study of poisons and toxins in the given drug molecules which leads to adverse and serious adverse reactions. Toxicology studies play the key role in Clinical Trials testing. Significant to investigate, interpret and communicate the toxic reactions Every Pharmacokinetic and Pharmacodynamic analysis are carried out in the clinical toxicology studies Includes the chemistry, Biology, biochemistry and the forensic sciences
Related: Clinical Trials Conferences| Clinical Trial Conferences| Pharmacovigilance Conferences | Clinical Trials Congress |ClinicalTrials Meetings | MedicalConferences | PharmacologyConferences | ClinicalResearch Conferences | Clinical research Society | Clinical Research Associations
Recommended: 9th World Congress on Clinical Pharmacyand Pharmacy Practice Amsterdam, Netherlands March 25-26, 2019, 4thInternational Conference onMedical Microbiology 2019 Vienna, Austria May 20-21, 2019, CPD Accredited8th European ClinicalMicrobiology and ImmunologyCongress Edinburgh, Scotland June 12-13, 2019, International Conference on Clinical Trials& Pharmacovigilance,Prague Czech Republic, September 23 -24 2019. World Congress on Biomarkers andClinical Research, Tokyo, Japan, August 5-6, 2019.
Related Associations: ThePharmaceutical Society of Australia (PSA), AustrianPharmaceutical Society (APS), KoreanResearch-based Pharmaceutical Industry Association (KRPIA), KuwaitPharmaceutical Association (KPA), IndianPharmaceutical Association (IPA).
Session on: Bioavailability & BioEquivalence
The amount of administered therapeutic medicine that is absorbed in site of drug action Blood concentrations of the active ingredients and their active metabolites will provide a marker for the concentration at the site of action with a valid measure of bioavailability Two drugs comprising identical active ingredients, otherwise two different dosage forms of the same investigational product possess similar bioavailability and produce the same effect at the site of action of Bioequivalence
Related: Clinical Trials Conferences| Clinical TrialConferences | Pharmacovigilance Conferences | Clinical Trials Congress |ClinicalTrials Meetings | MedicalConferences | PharmacologyConferences | ClinicalResearch Conferences | Clinical research Society | Clinical Research Associations
Recommended: 9th World Congress on Clinical Pharmacyand Pharmacy Practice Amsterdam, Netherlands March 25-26, 2019, 4thInternational Conference onMedical Microbiology 2019 Vienna, Austria May 20-21, 2019, CPD Accredited8th European ClinicalMicrobiology and ImmunologyCongress Edinburgh, Scotland June 12-13, 2019, International Conference on Clinical Trials& Pharmacovigilance,Prague Czech Republic, September 23 -24 2019. World Congress on Biomarkers andClinical Research, Tokyo, Japan, August 5-6, 2019.
Related Associations: BelgianSociety of Pharmaceutical Sciences (BGFW), EuropeanFederation for Pharmaceutical Sciences (EUFEPS),ItalianSociety for Pharmaceutical Sciences (SISF), Associationof the British Pharmaceutical Industry (ABPI), InternationalPharmaceutical Federation (FIP)
Session on: Phases of Clinical Trials
Clinicaltrials are conducted to gather knowledge relating to the security and effectuality of recent drug and device development. There are many steps andstages of approval within the clinical trials method before a drug or device may be sold within the client market
Phase I studies assess the security of a drug or device.
Phase II studies check the effectuality of a drug or device.
Phase III studies involve randomized and blind testing in many hundred to many thousand patients.
Phase IV studies, usually known as Post marketing Trials, are conducted once a drug or device has been approved for client sale.
Related: Clinical Trials Conferences| Clinical TrialConferences | Pharmacovigilance Conferences | Clinical Trials Congress |ClinicalTrials Meetings | MedicalConferences | PharmacologyConferences | ClinicalResearch Conferences | Clinical research Society | Clinical Research Associations
Recommended: 9th World Congress on Clinical Pharmacyand Pharmacy Practice Amsterdam, Netherlands March 25-26, 2019, 4thInternational Conference onMedical Microbiology 2019 Vienna, Austria May 20-21, 2019, CPD Accredited8th European ClinicalMicrobiology and ImmunologyCongress Edinburgh, Scotland June 12-13, 2019, International Conference on Clinical Trials& Pharmacovigilance,Prague Czech Republic, September 26 -27 2019. World Congress on Biomarkers andClinical Research, Tokyo, Japan, August 5-6, 2019.
Related Associations: ThePharmaceutical Society of Australia (PSA), AustrianPharmaceutical Society (APS), KoreanResearch-based Pharmaceutical Industry Association (KRPIA), KuwaitPharmaceutical Association (KPA), IndianPharmaceutical Association (IPA).
Session on: Interventionaland ObservationalStudies
Clinical study where the participants are identified as belonging to study groups and are evaluated for biomedical or health outcomes. Diagnostic, therapeutic, or other types of interventions are given to the patients, but the investigator does not allocate participants to a specific interventions/treatment. A focused study where Drugs,medical devices, procedures, vaccines, and other products that are either investigational or already available. Interventions can also include non-invasive approaches, such as education or modifying diet and exercise
Related: Clinical Trials Conferences| Clinical TrialConferences | Pharmacovigilance Conferences | Clinical Trials Congress |ClinicalTrials Meetings | MedicalConferences | PharmacologyConferences | ClinicalResearch Conferences | Clinical research Society | Clinical Research Associations
Recommended: 9th World Congress on Clinical Pharmacyand Pharmacy Practice Amsterdam, Netherlands March 25-26, 2019, 4thInternational Conference onMedical Microbiology 2019 Vienna, Austria May 20-21, 2019, CPD Accredited8th European ClinicalMicrobiology and ImmunologyCongress Edinburgh, Scotland June 12-13, 2019, International Conference on Clinical Trials& Pharmacovigilance,Prague Czech Republic, September 23 -24 2019. World Congress on Biomarkers andClinical Research, Tokyo, Japan, August 5-6, 2019.
Related Associations: CanadianSociety for Pharmaceutical Sciences (CSPS), PharmaceuticalManufacturers Association of Canada (PMAC),AmericanAssociation of Pharmacy Technicians (AAPT), AmericanSociety of Health-System Pharmacists (ASHYP)| SpanishSociety of Pharmaceutics and PharmaceuticalTechnology(SEFC).
Session on: Patient Centric Clinical Trials
Clinical trials struggle with both registration and patient retention. Scheming and running a study while keeping the patient viewpoint in mind can help at every stage of a clinical trial. There are several ways to put the patient first during each stage of a clinical trial, from staffing to retention to study follow-ups.
By Working with patient advocacy organizations.
By Using real-world data
Calculating the health literacy-of the patient
Site Accessibility
Related: Clinical Trials Conferences| Clinical TrialConferences | Pharmacovigilance Conferences | Clinical Trials Congress |ClinicalTrials Meetings | MedicalConferences | PharmacologyConferences | ClinicalResearch Conferences | Clinical research Society | Clinical Research Associations
Recommended: 9th World Congress on Clinical Pharmacyand Pharmacy Practice Amsterdam, Netherlands March 25-26, 2019, 4thInternational Conference onMedical Microbiology 2019 Vienna, Austria May 20-21, 2019, CPD Accredited8th European ClinicalMicrobiology and ImmunologyCongress Edinburgh, Scotland June 12-13, 2019, International Conference on Clinical Trials& Pharmacovigilance,Prague Czech Republic, September 23 -24 2019. World Congress on Biomarkers andClinical Research, Tokyo, Japan, August 5-6, 2019.
Related Associations: BelgianSociety of Pharmaceutical Sciences (BGFW), EuropeanFederation for Pharmaceutical Sciences (EUFEPS),ItalianSociety for Pharmaceutical Sciences (SISF), Associationof the British Pharmaceutical Industry (ABPI), InternationalPharmaceutical Federation (FIP)
Session on: Adaptive Clinical Trials
A Clinical Study protocol that agrees alterations to the trial and/or statistical measures of the trial after its initiation without undermining its legitimacy and integrity. The purpose is to make clinical trials more flexible, efficientand fast. Due to the level of flexibility involved, these trial designs are also termed as “flexibledesigns”.
Related: Clinical Trials Conferences| Clinical TrialConferences | Pharmacovigilance Conferences | Clinical Trials Congress |ClinicalTrials Meetings | MedicalConferences | PharmacologyConferences | ClinicalResearch Conferences | Clinical research Society | Clinical Research Associations
Recommended: 9th World Congress on Clinical Pharmacyand Pharmacy Practice Amsterdam, Netherlands March 25-26, 2019, 4thInternational Conference onMedical Microbiology 2019 Vienna, Austria May 20-21, 2019, CPD Accredited8th European ClinicalMicrobiology and ImmunologyCongress Edinburgh, Scotland June 12-13, 2019, International Conference on Clinical Trials& Pharmacovigilance,Prague Czech Republic, September 26 -27 2019. World Congress on Biomarkers andClinical Research, Tokyo, Japan, August 5-6, 2019.
Related Associations: ThePharmaceutical Society of Australia (PSA), AustrianPharmaceutical Society (APS), KoreanResearch-based Pharmaceutical Industry Association (KRPIA), KuwaitPharmaceutical Association (KPA), IndianPharmaceutical Association (IPA).
Session on: HIV Clinical Trials
Research and Trials towards the HIV medicine has been highly increased. Clinical Trials comprising study medicines to prevent HIV, Interventional trials that treat as well as prevent HIV and cohort studies that is based on the infections that is related to the HIV clinical Trials
Many governmental bodies and NGOorganizations works on underdeveloped and developing countries patients toparticipate in the ClinicalTrials studies
Related: Clinical Trials Conferences| Clinical TrialConferences | Pharmacovigilance Conferences | Clinical Trials Congress |ClinicalTrials Meetings | MedicalConferences | PharmacologyConferences | ClinicalResearch Conferences | Clinical research Society | Clinical Research Associations
Recommended: 9th World Congress on Clinical Pharmacyand Pharmacy Practice Amsterdam, Netherlands March 25-26, 2019, 4thInternational Conference onMedical Microbiology 2019 Vienna, Austria May 20-21, 2019, CPD Accredited8th European ClinicalMicrobiology and ImmunologyCongress Edinburgh, Scotland June 12-13, 2019, International Conference on Clinical Trials& Pharmacovigilance,Prague Czech Republic, September 23 -24 2019. World Congress on Biomarkers andClinical Research, Tokyo, Japan, August 5-6, 2019.
Related Associations: CanadianSociety for Pharmaceutical Sciences (CSPS), PharmaceuticalManufacturers Association of Canada (PMAC),AmericanAssociation of Pharmacy Technicians (AAPT), AmericanSociety of Health-System Pharmacists (ASHYP)| SpanishSociety of Pharmaceutics and PharmaceuticalTechnology(SEFC).
Session on: Clinical Trials in Cardiology
Clinical trials in cardiovascular drug shave big in size, scope, and complexity. As therapeutic approaches in cardiovascular drugs became simpler, the amount of proof needed to support progressive new advances has enhanced considerably. This fact, in conjunction with real and perceived will increase in regulative needs, and therefore the mandate for higher estimation of cardiovascular safety for non-cardiovascular therapies, have resulted in tumescent and prohibitively costly trials. Thus, despite the success of clinical trials in vessel drugs over the past twenty-five years, the power to bringnew cardiovascular therapies to patients would require new approaches to curtail value and maintain quality of future trials.
Related: Clinical Trials Conferences| Clinical TrialConferences | Pharmacovigilance Conferences | Clinical Trials Congress |ClinicalTrials Meetings | MedicalConferences | PharmacologyConferences | ClinicalResearch Conferences | Clinical research Society | Clinical Research Associations
Recommended: 9th World Congress on Clinical Pharmacyand Pharmacy Practice Amsterdam, Netherlands March 25-26, 2019, 4thInternational Conference onMedical Microbiology 2019 Vienna, Austria May 20-21, 2019, CPD Accredited8th European ClinicalMicrobiology and ImmunologyCongress Edinburgh, Scotland June 12-13, 2019, International Conference on Clinical Trials& Pharmacovigilance,Prague Czech Republic, September 23 -24 2019. World Congress on Biomarkers andClinical Research, Tokyo, Japan, August 5-6, 2019.
Related Associations: BelgianSociety of Pharmaceutical Sciences (BGFW), EuropeanFederation for Pharmaceutical Sciences (EUFEPS),ItalianSociety for Pharmaceutical Sciences (SISF), Associationof the British Pharmaceutical Industry (ABPI), InternationalPharmaceutical Federation (FIP)
Session on: Clinical Trials in Neurology
Neurology is a vast space encompassing various disease states, as well as multiple sclerosis, epilepsy, stroke and headache. These conditions influence people across the age spectrum and may be encountered all told apply settings. whereas neurology was once considered a sub specialty with few treatment choices,nowadays several therapies area unit obtainable which will considerably improve physical function and quality of life With a rise in old population can comeback a bigger prevalence of diseases of the aging brain, requiring clinicians to respond to the increasing demand for medicine care.1 annually, new studies area unit added to the prevailing literature pool all told of those numerous sub specialties, making a challenge for busy clinicians to stay up with best apply for his or her patients.
Related: Clinical Trials Conferences| Clinical TrialConferences | Pharmacovigilance Conferences | Clinical Trials Congress |ClinicalTrials Meetings | MedicalConferences | PharmacologyConferences | ClinicalResearch Conferences | Clinical research Society | Clinical Research Associations
Recommended: 9th World Congress on Clinical Pharmacyand Pharmacy Practice Amsterdam, Netherlands March 25-26, 2019, 4thInternational Conference onMedical Microbiology 2019 Vienna, Austria May 20-21, 2019, CPD Accredited8th European ClinicalMicrobiology and ImmunologyCongress Edinburgh, Scotland June 12-13, 2019, International Conference on Clinical Trials& Pharmacovigilance,Prague Czech Republic, September 23 -24 2019. World Congress on Biomarkers andClinical Research, Tokyo, Japan, August 5-6, 2019.
Related Associations: BelgianSociety of Pharmaceutical Sciences (BGFW), EuropeanFederation for Pharmaceutical Sciences (EUFEPS),ItalianSociety for Pharmaceutical Sciences (SISF), Associationof the British Pharmaceutical Industry (ABPI), InternationalPharmaceutical Federation (FIP)
Session on: Clinical Trials in Dermatology
Clinical trials are the backbone of contemporary day medication. Randomized, double blinded, placebo controlled studies are essential for advancement in medication and medicine. Skin conditions like psoriasis and dermatitis are among the foremost common health issues within the US and Most parts of the world. Clinical trials will give treatments that not solely supply objective enhancements in clinical disease status however also subjective improvements within the quality of lifetime of patients who square measure afflicted with the disease.Significance towards the dermatology trials are gaining rapid attention, these trials tend to build confidence to the people who are affected with skin diseases and syndromes
Related: Clinical Trials Conferences| Clinical TrialConferences | Pharmacovigilance Conferences | Clinical Trials Congress |ClinicalTrials Meetings | MedicalConferences | PharmacologyConferences | ClinicalResearch Conferences | Clinical research Society | Clinical Research Associations
Recommended: 9th World Congress on Clinical Pharmacyand Pharmacy Practice Amsterdam, Netherlands March 25-26, 2019, 4thInternational Conference onMedical Microbiology 2019 Vienna, Austria May 20-21, 2019, CPD Accredited8th European ClinicalMicrobiology and ImmunologyCongress Edinburgh, Scotland June 12-13, 2019, International Conference on Clinical Trials& Pharmacovigilance,Prague Czech Republic, September 23 -24 2019. World Congress on Biomarkers andClinical Research, Tokyo, Japan, August 5-6, 2019.
Related Associations: BelgianSociety of Pharmaceutical Sciences (BGFW), EuropeanFederation for Pharmaceutical Sciences (EUFEPS),ItalianSociety for Pharmaceutical Sciences (SISF), Associationof the British Pharmaceutical Industry (ABPI), InternationalPharmaceutical Federation (FIP)
Session on: ClinicalTrials in Oncology
Oncology trials have given life and strength to the cancer survivors, over lakhs of people are benefitted from oncology trials the death rate has been slightly decreased and givinga promising future to the oncology studies Clinical Trials 2019 takes a strongstand in invites the researchers to platform their research in oncology trials
Related: Clinical Trials Conferences| Clinical TrialConferences | Pharmacovigilance Conferences | Clinical Trials Congress |ClinicalTrials Meetings | MedicalConferences | PharmacologyConferences | ClinicalResearch Conferences | Clinical research Society | Clinical Research Associations
Recommended: 9th World Congress on Clinical Pharmacyand Pharmacy Practice Amsterdam, Netherlands March 25-26, 2019, 4th International Conference onMedical Microbiology 2019 Vienna, Austria May 20-21, 2019, CPD Accredited8th European ClinicalMicrobiology and ImmunologyCongress Edinburgh, Scotland June 12-13, 2019, International Conference on Clinical Trials& Pharmacovigilance,Prague Czech Republic, September 23 -24 2019. World Congress on Biomarkers andClinical Research, Tokyo, Japan, August 5-6, 2019.
Related Associations: BelgianSociety of Pharmaceutical Sciences (BGFW), EuropeanFederation for Pharmaceutical Sciences (EUFEPS),ItalianSociety for Pharmaceutical Sciences (SISF), Associationof the British Pharmaceutical Industry (ABPI), InternationalPharmaceutical Federation (FIP)
Session on: Pharmacovigilance
The pharmacological science relating to the detection, assessment, monitoring the safety of the pharmaceutical drug or medical device, and prevention of adverse effects with other pharmaceutical products after being released from the market is an important part of pharmacovigilance. Monitoring the adverse reactions and trials related events plays a vital role in the Pharmacovigilance practices. Data obtained from the patients and healthcare providers through the pharmacovigilance agreements (PVAs), medical literature, plays a critical role in providing the data necessary forpharmacovigilance to take place
Related: Clinical Trials Conferences| Clinical TrialConferences | Pharmacovigilance Conferences | Clinical Trials Congress |ClinicalTrials Meetings | MedicalConferences | PharmacologyConferences | ClinicalResearch Conferences | Clinical research Society | Clinical Research Associations
Recommended: 9th World Congress on Clinical Pharmacy and Pharmacy Practice Amsterdam, Netherlands March 25-26, 2019, 4th International Conference on Medical Microbiology 2019 Vienna, Austria May 20-21, 2019, CPD Accredited8th European ClinicalMicrobiology and ImmunologyCongress Edinburgh, Scotland June 12-13, 2019, International Conference on Clinical Trials& Pharmacovigilance,Prague Czech Republic, September 23 -24 2019. World Congress on Biomarkers andClinical Research, Tokyo, Japan, August 5-6, 2019.
Related Associations: ThePharmaceutical Society of Australia (PSA), AustrianPharmaceutical Society (APS), KoreanResearch-based Pharmaceutical Industry Association (KRPIA), KuwaitPharmaceutical Association (KPA), IndianPharmaceutical Association (IPA).
Session on: GoodPharmacovigilance practices
After the thalidomide tragedy, many union bodies put forth the set of regulations to abide the Ethical and social aspects of drug use. Good pharmacovigilance practices (GVP) are a set of events which are established to facilitate the performance of pharmacovigilance in the EU. Encourage a safe, rational andeffective use of medicines. These practices provided the enhancement of patient safety and care while using medicines, drugs or any medical interventions.
Related: Clinical Trials Conferences| Clinical TrialConferences | Pharmacovigilance Conferences | Clinical Trials Congress |ClinicalTrials Meetings | MedicalConferences | PharmacologyConferences | ClinicalResearch Conferences | Clinical research Society | Clinical Research Associations
Recommended: 9th World Congress on Clinical Pharmacyand Pharmacy Practice Amsterdam, Netherlands March 25-26, 2019, 4thInternational Conference onMedical Microbiology 2019 Vienna, Austria May 20-21, 2019, CPD Accredited8th European ClinicalMicrobiology and ImmunologyCongress Edinburgh, Scotland June 12-13, 2019, International Conference on Clinical Trials& Pharmacovigilance,Prague Czech Republic, September 23 -24 2019. World Congress on Biomarkers andClinical Research, Tokyo, Japan, August 5-6, 2019.
Related Associations: CanadianSociety for Pharmaceutical Sciences (CSPS), PharmaceuticalManufacturers Association of Canada (PMAC),AmericanAssociation of Pharmacy Technicians (AAPT), AmericanSociety of Health-System Pharmacists (ASHYP)| SpanishSociety of Pharmaceutics and PharmaceuticalTechnology(SEFC).
Session on: DrugSafety
Randomizedcontrolled trials are the principal suggests that of creating the efficaciousness of drugs. However, pre-marketing trials are restricted in size and length and exclude risky populations. They have restricted applied math power to discover rare however doubtless serious adverse events in real-world patients. We summarize the principal method challenges within the coverage,analysis and interpretation of safety information in clinical trials exploitation recent examples from systematic reviews. These challenges embrace the shortage of an evidentiary gold normal, the restricted applied math power of irregular controlled trials and ensuing sort a pair of error, the shortageof adequate ascertainment of adverse events and restricted generalization of trials that exclude high risk patients.
Related: Clinical Trials Conferences| Clinical TrialConferences | Pharmacovigilance Conferences | Clinical Trials Congress |ClinicalTrials Meetings | MedicalConferences | PharmacologyConferences | ClinicalResearch Conferences | Clinical research Society | Clinical Research Associations
Recommended: 9th World Congress on Clinical Pharmacyand Pharmacy Practice Amsterdam, Netherlands March 25-26, 2019, 4thInternational Conference onMedical Microbiology 2019 Vienna, Austria May 20-21, 2019, CPD Accredited8th European ClinicalMicrobiology and ImmunologyCongress Edinburgh, Scotland June 12-13, 2019, International Conference on Clinical Trials& Pharmacovigilance,Prague Czech Republic, September 23 -24 2019. World Congress on Biomarkers andClinical Research, Tokyo, Japan, August 5-6, 2019.
Related Associations: BelgianSociety of Pharmaceutical Sciences (BGFW), EuropeanFederation for Pharmaceutical Sciences (EUFEPS),ItalianSociety for Pharmaceutical Sciences (SISF), Associationof the British Pharmaceutical Industry (ABPI), InternationalPharmaceutical Federation (FIP)
Session on: ClinicalTrials Auditing
A methodical check-up, by people not directly involved in the trial, to estimate whether the implementation, data recording and analysis of the trial are in accordance with the trial proposal, standard operation regulations and other guidelines linked to drugclinical trials. Drug supervision and management authorities will official evaluate the documents, implement, record and other aspects of a clinicaltrial. Inspection can take place in the trial institute, location of the sponsor, or venue of the contract research organization (CRO)
Related: Clinical Trials Conferences| Clinical TrialConferences | Pharmacovigilance Conferences | Clinical Trials Congress |ClinicalTrials Meetings | MedicalConferences | PharmacologyConferences | ClinicalResearch Conferences | Clinical research Society | Clinical Research Associations
Recommended: 9th World Congress on Clinical Pharmacyand Pharmacy Practice Amsterdam, Netherlands March 25-26, 2019, 4thInternational Conference onMedical Microbiology 2019 Vienna, Austria May 20-21, 2019, CPD Accredited8th European ClinicalMicrobiology and ImmunologyCongress Edinburgh, Scotland June 12-13, 2019, International Conference on Clinical Trials& Pharmacovigilance,Prague Czech Republic, September 23 -24 2019. World Congress on Biomarkers andClinical Research, Tokyo, Japan, August 5-6, 2019.
Related Associations: ThePharmaceutical Society of Australia (PSA), AustrianPharmaceutical Society (APS), KoreanResearch-based Pharmaceutical Industry Association (KRPIA), KuwaitPharmaceutical Association (KPA), IndianPharmaceutical Association (IPA).
Session on: BiomedicalClinical Trials
Clinical trials are prospective biomedical or activity analysis studies of human subjects that area unit designed to answer specific questions about medicine or behavioral interventions (drugs, treatments, devices, or new ways in which in which of using known drugs, treatments, or devices). Clinical trials are accustomed confirm whether new biomedical or behavioral interventions are safe, efficacious, and effective. Biomedical clinicaltrials of an experimental drug, treatment, device, or behavioural Intervention might proceed through Tests a replacement biomedical intervention during a little cluster of individuals, Study the biomedical or behavioral lintervention during a larger cluster of individuals, Study to see effectualness of the medicine or activity intervention in giant teams of individuals, Studies conducted when the intervention has been marketed. These studies are designed to observe the effectiveness of the approved intervention among the ultimate population and to gather info about any adverse effects related to widespread use.
Related: Clinical Trials Conferences| Clinical Trial Conferences| Pharmacovigilance Conferences | Clinical Trials Congress |ClinicalTrials Meetings | MedicalConferences | PharmacologyConferences | ClinicalResearch Conferences | Clinical research Society | Clinical Research Associations
Recommended: 9th World Congress on Clinical Pharmacyand Pharmacy Practice Amsterdam, Netherlands March 25-26, 2019, 4thInternational Conference onMedical Microbiology 2019 Vienna, Austria May 20-21, 2019, CPD Accredited8th European Clinical Microbiologyand Immunology CongressEdinburgh, Scotland June 12-13, 2019, International Conference on Clinical Trials& Pharmacovigilance,Prague Czech Republic, September 23 -24 2019. World Congress on Biomarkers andClinical Research, Tokyo, Japan, August 5-6, 2019.
Related Associations: CanadianSociety for Pharmaceutical Sciences (CSPS), PharmaceuticalManufacturers Association of Canada (PMAC),AmericanAssociation of Pharmacy Technicians (AAPT), AmericanSociety of Health-System Pharmacists (ASHYP)| SpanishSociety of Pharmaceutics and PharmaceuticalTechnology(SEFC).
Session on: EthicalCommittee and RegulatoryBoard
Institutional Review Board (IRB)whose primary mandate is to shield the rights and safeguard the welfare of the topics. In their deliberations, IRBs square measure expected to require intoconsideration the character, content, and style of the analysis and make sure that they're compliant with the moral principles of the dirt track Report,Declaration of national capital, moral Principles of medical specialty analysis(Indian council of medical analysis (ICMR), 2006)[1] and, once applicable and applicable, the regulative needs of Country.
ECs reassure the general public that the rights and welfare of human subject’s square measure seriously thought of by folks that don't have an unconditional interest within the outcome of the analysis.
Related: Clinical Trials Conferences| Clinical TrialConferences | Pharmacovigilance Conferences | Clinical Trials Congress |ClinicalTrials Meetings | MedicalConferences | PharmacologyConferences | ClinicalResearch Conferences | Clinical research Society | Clinical Research Associations
Recommended: 9th World Congress on Clinical Pharmacyand Pharmacy Practice Amsterdam, Netherlands March 25-26, 2019, 4thInternational Conference onMedical Microbiology 2019 Vienna, Austria May 20-21, 2019, CPD Accredited8th European ClinicalMicrobiology and ImmunologyCongress Edinburgh, Scotland June 12-13, 2019, International Conference on Clinical Trials& Pharmacovigilance,Prague Czech Republic,September 23 -24 2019. World Congress on Biomarkers andClinical Research, Tokyo, Japan, August 5-6, 2019.
Related Associations: CanadianSociety for Pharmaceutical Sciences (CSPS), PharmaceuticalManufacturers Association of Canada (PMAC),AmericanAssociation of Pharmacy Technicians (AAPT), AmericanSociety of Health-System Pharmacists (ASHYP)| SpanishSociety of Pharmaceutics and PharmaceuticalTechnology(SEFC).
Session on: ArtificialIntelligence in ClinicalTrials
Man-madebrainpower (AI) innovations have made huge advances and a stream of companiesare focusing, applying these advances to business challenges and, all thewhile, pioneering new trails. While numerous individuals might considercomputerized reasoning as something recondite and in the domain of sci-fi,there are genuine applications that can offer organizations some assistancewith solving complex issues, for example, understanding enormous information,increasing human choice making, or furnishing clients with master guidance.AIadvancements are accessible for different markets and parts, and offer fiveconsumable business abilities. A third region is an instinctive correspondence,going past basic voice acknowledgment and characteristic dialect handling,looking to permit the catch of the genuine importance at the semantics ofcontent and discourse, by mapping outward appearances and signals toenthusiastic state and mapping voice inflection to passionate state.
Related: Clinical Trials Conferences| Clinical TrialConferences | Pharmacovigilance Conferences | Clinical Trials Congress |ClinicalTrials Meetings | MedicalConferences | PharmacologyConferences | ClinicalResearch Conferences | Clinical research Society | Clinical Research Associations
Recommended: 9th World Congress on Clinical Pharmacyand Pharmacy Practice Amsterdam, Netherlands March 25-26, 2019, 4thInternational Conference onMedical Microbiology 2019 Vienna, Austria May 20-21, 2019, CPD Accredited8th European ClinicalMicrobiology and ImmunologyCongress Edinburgh, Scotland June 12-13, 2019, International Conference on Clinical Trials& Pharmacovigilance,Prague Czech Republic, September 23 -24 2019. World Congress on Biomarkers andClinical Research, Tokyo, Japan, August 5-6, 2019.
The successful series of Breast Cancer Congress is taking the Privilege to welcome you to 9th European Congress on Breast Cancer, Women’s Health & Therapeutics, Paris France scheduled in the month of February from 25-26, 2019.
The Scientific Sessions are designed by experts and researchers from all around the world who will be showcasing their research and expertise in the field of Breast Cancer and Women Health.
We gather researchers to reveal, examine and revise the methods,techniques and research developing in the scientific community, making it accessible throughout the world even after the event.
The successful 8th World Congress on Breast Cancer Management & Therapy created an awesome impact by networking experts from East Asia to UK. 50000 visitors visited and submitted their work and broke down to meet and explore the prestigious event Breast Cancer 2018 at London, UK.
The 9th European Congress is setting it’s platform in Paris to host and bring the researchers of Breast Cancer and Women Health under one roof and provide opportunities to Young Researchers, meet and socialize with the experts and network all around the globe.
We welcome you to start off your journey with us at 9th European Congress on Breast Cancer, Women's Health& Therapeutics in the City of Lights-Paris, France
Why to Attend.
Showcase your recent research
Keynote sessions by emeritus of renowned Universities and Industries
Strengthen your research opportunities with the experts.
Collaborations with the Med Kings of the field
Opportunity to meet highly eminent Speakers and Sponsorship.
Poster, e-poster, exhibiting and speaker sessions which can be accessed even after the conference.
e global pharmacovigilance (PV) market is anticipated to succeed in USD 10.27 billion by 2025, per a replacement report by Grand read analysis, Inc. The market is anticipated to witness growth at 13.1% CAGR because of Increasing incidence of ADR is vital driver for the expansion of pharmacovigilance market. As of 2015, the U.S. government agency received roughly 253,017 serious adverse events and 44,693 deaths related to adverse drug reactions (ADRs). This shows the potential demand for implementing safety and pharmacovigilance services over the forecast amount
Related: Clinical Trials Conferences | Clinical Trial Conferences | Pharmacovigilance Conferences | Clinical Trials Congress | Clinical Trials Meetings | Medical Conferences | Pharmacology Conferences | Clinical Research Conferences | Clinical research Society | Clinical Research Associations
Related Associations: Belgian Society of Pharmaceutical Sciences (BGFW), European Federation for Pharmaceutical Sciences (EUFEPS),Italian Society for Pharmaceutical Sciences (SISF), Association of the British Pharmaceutical Industry (ABPI), International Pharmaceutical Federation (FIP)
According to World Health Organization’s (WHO) report on prescribed drugs consumption, medicines to treat chronic diseases accounted for a bigger proportion of the whole volume of drug consumption in non-hospital set ups. because of this, there has been a major rise within the variety of medicines created offered to aid customers. Rising demand for medicine has considerably heightened the necessity for novel medicine development via intensive clinical trials, that is any expected to serve this market with profitable opportunities.
The company’s area unit endeavor strategic initiatives like collaboration with the PV service suppliers to urge access to medical data and manage PV workflows
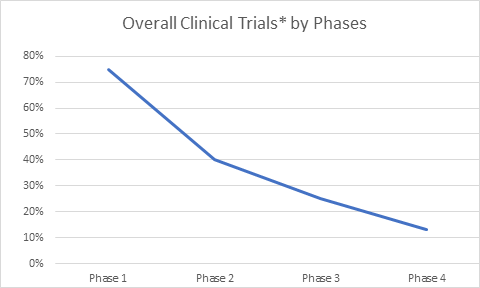
Clinical Trials Market has been calculable at USD17.12 Billion in 2018 and is projected to achieve USD24.87 Billion by 2023 at a CAGR of seven.75% throughout the forecast amount from 2018 to 2023 Clinical Trials area unit analysis studies performed on humans to achieve specific data concerning medicine interventions like novel vaccines, devices, treatments and medicines and thereby generating safety knowledge. they're conducted in four phases specifically, Phase I, II, III and IV. it's necessary for additional approval of the drug and to bring it into the market.
Related: Clinical Trials Conferences | Clinical Trial Conferences | Pharmacovigilance Conferences | Clinical Trials Congress | Clinical Trials Meetings | Medical Conferences | Pharmacology Conferences | Clinical Research Conferences | Clinical research Society | Clinical Research Associations
Related Associations: Belgian Society of Pharmaceutical Sciences (BGFW), European Federation for Pharmaceutical Sciences (EUFEPS),Italian Society for Pharmaceutical Sciences (SISF), Association of the British Pharmaceutical Industry (ABPI), International Pharmaceutical Federation (FIP)
The marketplace for Clinical Trials is principally driven because of advancement in technology, increasing demand of innovative solutions in aid trade, and more boosted the alliances between the pharma-biotech corporations and therefore the clinical analysis organizations. But the market growth may be hampered because of factors like lack of masterly professionals to control advanced CTMS solutions and budget constraints of small- and medium-sized pharmaceutical and biotechnology corporations and tiny CROs.
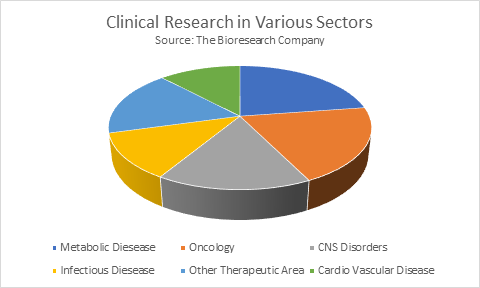
Global marketplace for Clinical Trials is segmental supported section, style and indication. By Phase, the market is more sub-segmented into clinical test, Phase II, Phase III. By design, the market is more sub-segmented into Interventional trials, empirical trials, distended Access trials. By indication, the market is more sub-segmented into reaction, Blood disorders, Cancer, Circulatory, CNS, Congenital, CVS, medical specialty, Ear, channel, gu, Infections, Mental disorders, Metabolic, system, Nose, medical specialty. Cancer and systema nervosum centrale area unit the biggest market share contributors to the market. Segments like channel, Circulatory, medical specialty and Mental Disorders area unit moving with high CAGR because of their increasing prevalence.
Related: Clinical Trials Conferences | Clinical Trial Conferences | Pharmacovigilance Conferences | Clinical Trials Congress | Clinical Trials Meetings | Medical Conferences | Pharmacology Conferences | Clinical Research Conferences | Clinical research Society | Clinical Research Associations
Related Associations: Belgian Society of Pharmaceutical Sciences (BGFW), European Federation for Pharmaceutical Sciences (EUFEPS),Italian Society for Pharmaceutical Sciences (SISF), Association of the British Pharmaceutical Industry (ABPI), International Pharmaceutical Federation (FIP)
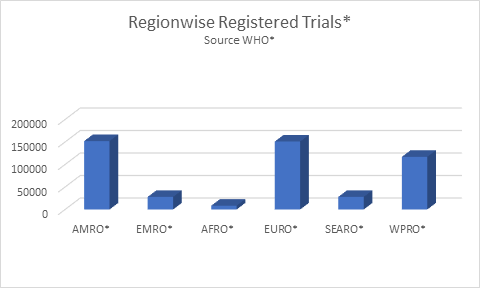
Geographically, world marketplace for Clinical Trials is segmental into North America, Asia Pacific, geographical area, Europe, and Middle-East and continent. North America is that the biggest market in terms of market share across the world, followed by Europe. Ample convenience of funds to source Clinical Trials is the most important growth driver for the North America. The unfold of analytics and awareness of its potential area unit increasing at a fast rate in Europe. The Clinical Trials market within the Asia-Pacific region is projected to grow at the best CAGR, that is greatly because of initiatives from the govt. and contributions from tutorial laboratories, that helped augment growth.
Why Czech Republic?
Among European countries, the European country is presently one in every of the foremost dominant run markets. With a population of roughly 10.5 million, reportedly sensible compliance to ICH-GCP tips, and therefore the presence of extremely qualified clinical investigators, the European country offers decent surroundings to perform clinical trials.
The European country has the third highest large intestine cancer incidence rate within the world and one the best excretory organ and carcinoma incidence within the EU.
There are over 800 sites within the European country, each public and personal hospitals and clinics, totally equipped and providing the best normal of treatment and diagnostic procedures. web site density is that the highest one in European region and accounts for seventy-seven (number of websites per one million population).
Investigators are ICH GCP practised, well educated, motivated and deliver terribly prime quality information.
Most of the Czech employees have scientific background, that results in glorious relations with the investigators and prime quality of knowledge.
Related: Clinical Trials Conferences | Clinical Trial Conferences | Pharmacovigilance Conferences | Clinical Trials Congress | Clinical Trials Meetings | Medical Conferences | Pharmacology Conferences | Clinical Research Conferences | Clinical research Society | Clinical Research Associations
Related Associations: Belgian Society of Pharmaceutical Sciences (BGFW), European Federation for Pharmaceutical Sciences (EUFEPS),Italian Society for Pharmaceutical Sciences (SISF), Association of the British Pharmaceutical Industry (ABPI), International Pharmaceutical Federation (FIP)
irstly, we would like to thank all our wonderful keynotes, speakers, conference attendees, students, associations, media partners, exhibitors and guests for making Clinical Trials 2019 a successful and splendid event and we are overwhelmed by their responses.
Allied Academies hosted the joint event of “International Conferenceon Clinical Trials & Pharmacovigilance” during February 28- March 01, 2019 at Holiday Inn Paris – Marne La Vallée 2 boulevard du Levant, 93160 Noisy-le-grand, Paris, France with the theme “Exploring recent trends in Clinical Trials & Research”. Benevolent response and active participation were received from the Editorial Board Members of supporting International Journals as well as from the leading academic scientists, researchers, research scholars, students and leaders from the fields of Clinical Trials & Pharmacovigilance who made this event successful.
The conference was marked by the attendance of young and brilliant researchers, business delegates and talented student communities representing more than 18 countries, who have driven this event into the path of success. The conference highlighted through various sessions on current retroviral research.
Clinical Trials 2019 witnessed an amalgamation of peerless speakers who enlightened the crowd with their knowledge and confabulated on various new-fangled topics related to the fields of Clinical Trials, Pharmacology, Palliative medicine, Gynecology and Stroke
The meeting was carried out through various sessions, in which the discussions were held on the following major scientific tracks:
Pre-Clinical Assessment
Clinical Trials
Phases of Clinical Trials
Interventional Clinical Trials
Patient Identification
Quality of Life in Clinical Trials
Clinical data managements
Inhuman Clinical Trials
Veterinary Clinical Trials
Drug design and Safety
Clinical Trials in Various Disease
Clinical trials in Oncology
Medical Device Research
Pharmacovigilance’s
Identifying the Ethical Challenges
Ethics in Clinical Trials
Future in Clinical Trials
The conference proceedings were carried out through various Scientific-sessions and plenary lectures. The conference was embarked with an opening ceremony followed by a series of lectures delivered by both Honourable Guests and members of the Keynote forum. The adepts who promulgated the theme with their exquisite talk were;
Seongtae Bae| University of South Carolina| USA
Vesna Popovska | BC Children’s Hospital | Canada
Danielle IWANDZA| PharmaCqARE| France
Abdullah Altamimi| KFMC| Saudi Arabia
Mariana C M| Sao Paulo University | Brazil
The event spotlighted various areas of Clinical Trials & Pharmacovigilance along with Workshops and plenary lectures from the speakers of various universities and organizations like
Our Collaborator: Neo Nano Medics USA | South Korea
University of South Carolina | USA
BC Children’s Hospital | Canada
KFMC| Saudi Arabia
Hospital Juan Ramón Jiménez | Spain
Sao Paulo University | Brazil
Johnson & Johnson | Brazil
EDCTP | The Netherlands
Nizwa Hospital | Oman
lyceum of the Philippines university | Philippines
CIPLA | India
Baba Farid University of Health Sciences | India
Foundation de Beaumont Bonelli for cancer research | Italy
High Point Clinical Trials Centre | USA
CNS Ratings LLC | USA
Health Unlocked | United Kingdom
We are also obliged to various delegate experts, company representatives and other eminent personalities who supported the conference by facilitating active discussion forums. We sincerely thank the Organizing Committee Members for their gracious presence, support, and assistance towards the success of Clinical Trials 2019.